-
FDA’s Agentic AI Moment: Why 2026 Will Redefine Regulatory Review
Let’s talk about a quiet but massive shift happening at the FDA. It’s moving from just experimenting with AI to fully embedding it into the heart of how it reviews medical products. For anyone in medtech, 2026 is the year this change becomes real. Think of it this way: the FDA is moving from having…
-
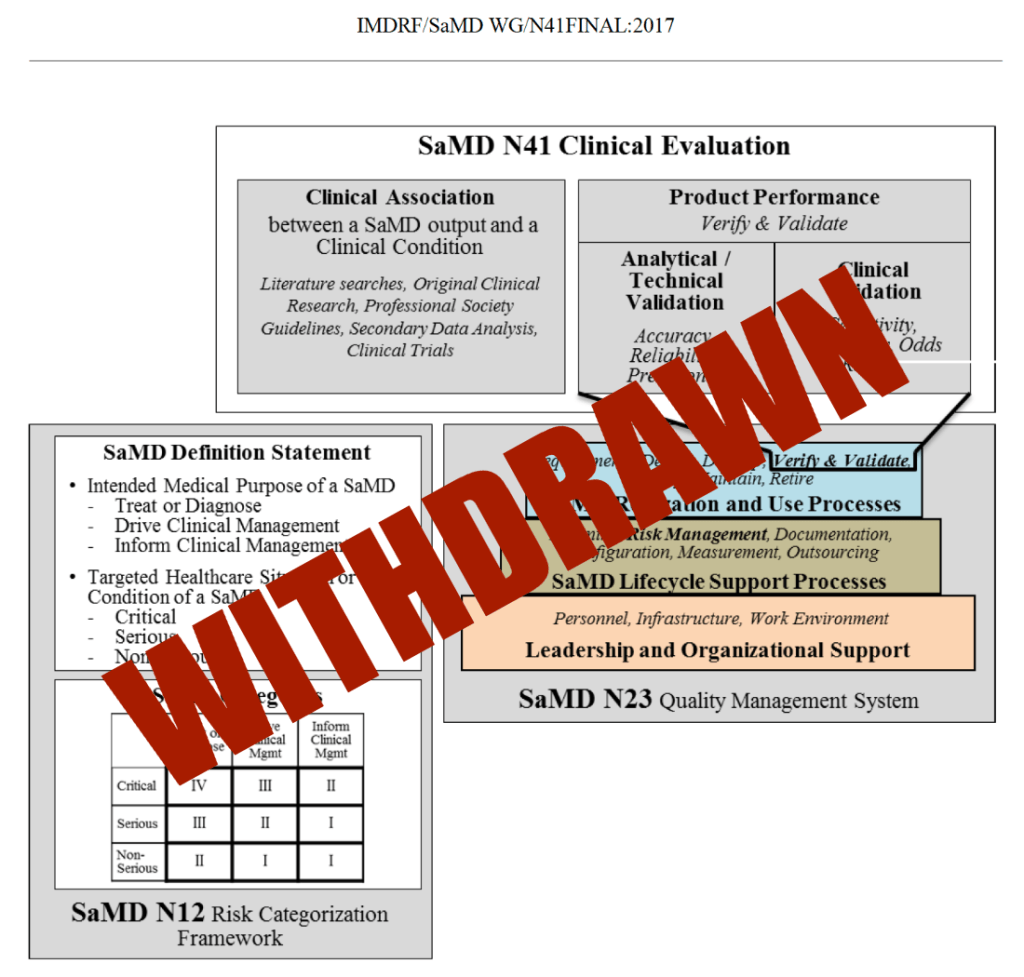
FDA Just Pulled the SaMD Clinical Evaluation Guidance. Here’s What That Really Means in 2026.
By Leon Zheng, Co-founder & CEO So… FDA quietly killed one of the anchor documents for SaMD evidence strategy, and a lot of people are still acting like nothing happened. If you build or regulate SaMD, this is not a “meh” housekeeping change. What actually happened FDA formally withdrew the guidance “Software as a Medical…
-
SE.ai Joins SCbio: Advancing Life Sciences in South Carolina
We are thrilled to announce that SE.ai has been accepted into SCbio, South Carolina’s premier life sciences organization dedicated to fostering innovation and growth in the industry. This milestone marks an exciting step forward for us as we join a vibrant community committed to transforming healthcare and improving lives through science and technology. SCbio’s mission…
-
Navigating the 510(k) Premarket Notification Process: A Deep Dive into Predicate Device Selection
By Leon Zheng, Co-founder & CSO Introduction The U.S. Food and Drug Administration (FDA) plays a critical role in ensuring the safety and efficacy of medical devices entering the market. Among the regulatory pathways available, the 510(k) premarket notification process is the most commonly used, accounting for over 80% of FDA medical device clearances…
-
SE.ai joins SCRA as a member company
From https://www.scra.org/scra-announces-new-member-companies-and-grant-funding-mar25/ SCRA Announces New Member Companies and Grant Funding Columbia, S.C. – LCIX, Matty AI, and SE.ai were accepted as South Carolina Research Authority Member Companies. Agricarbon US, Bio Streamline, Blotting Innovations, Green Cape Health, and Lumiq received new grant funding. All SCRA Member Companies receive coaching and access to SCRA’s Member Benefits and Startup Resources, can apply for…
Articles & events
SE.ai is currently accepting medtech partners for all products.
To apply or secure your spot on our waitlist, please fill out our partner inquiry form.
A limited number of Partners are chosen per program.